Oxidation states and ionicity
Abstract
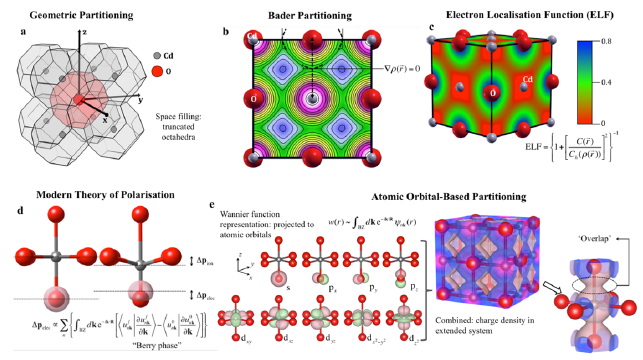
The concepts of oxidation state and atomic charge are entangled in modern materials science. We distinguish between these quantities and consider their fundamental limitations and utility for understanding material properties. We discuss the nature of bonding between atoms and the techniques that have been developed for partitioning electron density. While formal oxidation states help us count electrons (in ions, bonds, lone pairs), variously defined atomic charges are usefully employed in the description of physical processes including dielectric response and electronic spectroscopies. Such partial charges are introduced as quantitative measures in simple mechanistic models of a more complex reality, and therefore may not be comparable or transferable. In contrast, oxidation states are defined to be universal, with deviations constituting exciting challenges as evidenced in mixed-valence compounds, electrides and highly correlated systems. This Perspective covers how these concepts have evolved in recent years, our current understanding and their significance.
A fun perspective to be part of! It involved reading a lot of interesting older papers on electronic structure and bonding in condensed matter. We show that the concept of oxidation state has survived intact and is still very useful heuristically. There are exceptions, which we discuss, and future challenges.